Investigating Alternative ISRU Derived Fuels for Lunar Launch
The following is me sharing one of my last projects from graduate school where I looked into what kinds of fuels that could be made from local resources on the moon, aside from water derived options (as I wanted to see what potential other fuels might provide)
The international scientific community has shown tremendous interest in establishing one or more lunar research outposts that would serve as a research and development resource that would invaluably expand humanity’s ability to understand the universe. For the long-term operation of lunar research facilities to be as robust as possible, the ability to utilize local resources will be paramount. Reducing the need for imported fuels and launch systems will increase the ability for a lunar base to provide more than scientific knowledge as a return on investment.
In Situ Resource Utilization has been a topic of NASA research for decades. Initiatives to develop propellants from what can be found on the lunar surface has been key to this research. While propellants derived from extracted water ice garner the largest portion of pop-culture interest, the relatively small amounts of water, in tandem with the importance of water to human crews, developing other means of launching lunar payloads that conserve water will be of tremendous import.
An ISRU launch solution can come in many forms, from gas guns, centrifugal launchers, refueling of Earth made re-usable launchers, and fully locally built launch systems. The relative mechanical simplicity of gas guns and centrifugal launchers make them likely solutions for payloads capable of surviving 100s-1000s of Gees. Providing oxidizers and fuel for a re-usable space craft will likely be the most immediate ISRU launch solution developed on the lunar surface as it “only” requires the ability to refine local chemistry[1]. This paper will focus on the creation of engine/booster elements that can be made locally and reliably provide thrust for payloads leaving the lunar surface.
To that end whatever engine design is selected should emphasize manufacturability, both of propellants and engines and providing reliable thrust.
With the evolving landscape of additive manufacturing in both precision and material selection[2] as dynamic as it is, this paper will consider any potential mechanical design may be built. Those designs that emphasize fewer moving parts will be given a higher score based on the premise that fewer moving parts will require less time to fabricate. Any future research should account for technology readiness levels of available manufacturing techniques and rescore designs accordingly. In 2015 NASA had validated the ability of 3-D printed combustion chambers of surviving temperatures more than 3500K[3][4], an
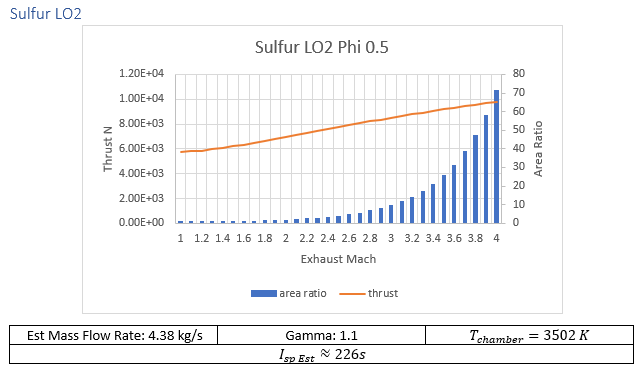
Lunar regolith is a fine jagged dust with only the faintest of traces of hydrogen and carbon[5], elements critical to human life. What regolith has in true abundance is oxygen, silicon, and metallic elements. To that end NASA Glenn Research Center’s Chemical Equilibrium with Applications (CEA) was used to investigate fuel chemistries might be able to provide lift. Beyond silicon and metallic elements, sulfur was added for consideration after finding reference to brimstone rockets during research. While sulfur makes up less than 0.2% of lunar regolith by mass, its ability to operate as a liquid at reasonable storage temperatures made it worth considering.
Of the above potential chemical reagents, Iron and Calcium showed no useful combustion properties in CEA. Silicon and silane showed the ability to produce a useful exothermic reaction, unfortunately silicon’s melting point over 1650 K would make storing the liquid difficult[6]. Silane while liquid at useful temperatures suffers from requiring extracted hydrogen, a non-starter for the parameters given.
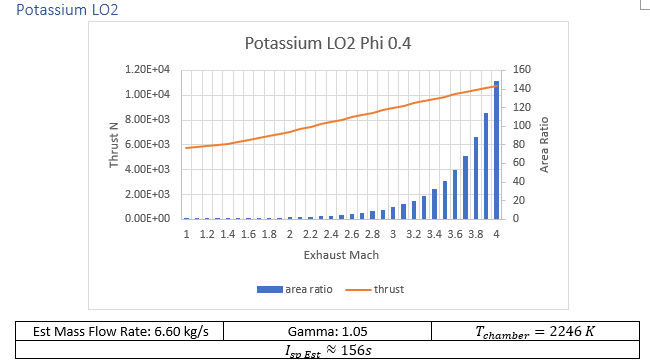
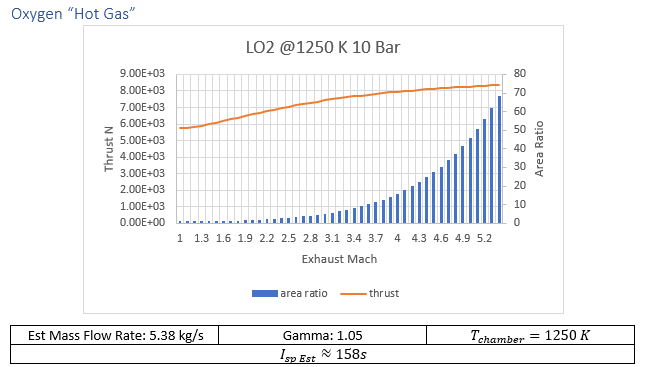
Aluminum, sulfur, potassium, all show potential as plentiful fuel sources. Two additional options that will be considered are a “simple” cold gas thruster and a thermally enhanced “cold gas” oxygen engine.
To aid in more direct comparisons, our engine properties will do their best to follow those of the RS-18 engine as a general reference. The RS-18 was used as the ascent engine for the Apollo Lunar Module. As a pressure fed system using NTO/Aerozine-50, the RS-18 was capable of producing ~15.6 kN of thrust[7]. With a diameter of roughly 0.8 cm and a bell length of about 1.3 meters we will use similar geometric constraints. Using the maximum outer diameter of the RS-18, a throat radius of 0.05m was estimated using Rao’s 15 deg half angle cone[8].
Chamber pressures the original RS-18 engines had a chamber pressure of 120[9] psia (827kPa or 8.27 Bar). For this analysis a chamber pressure of 10 Bar has been used.
Oxidizer Fuel weight ratios with the highest specific impulse provided from CEA were used in calculations, tabulated data can be found in the additional materials.
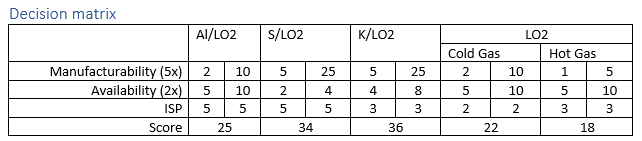
While the aluminum LOx engine has relatively strong performance with an estimated thrust of almost 10 kN in the theoretical model, experimental results were less positive. For the engine design to work, micro-particles of aluminum dust must be pushed into the reaction chamber, the challenge of making both a hopper for the aluminum powder and the actual aluminum particles themselves the aluminum engine is unlikely to meet our needs.
At time of writing robust tools for the analysis of how heating exhaust gases after leaving the throat of a cold gas thruster were beyond the ability of this paper’s author. Research similar heating cryonics for cold gas thrusters[10] and laser enhanced rocket propulsion[11], represent an opportunity for launch solution that is locally mechanically very simple, with much of the complexity offloaded to remote power sources.
Conclusion
Given the requirements outlined above the so-called brimstone rocket lost out to potassium/liquid oxygen. While sulfur oxygen reaction gives an additional 50% ISP over potassium oxidizing, the increased chemical availability of potassium gives it higher interest. At time of writing the only mention that the author was able to find associated with using potassium as a propellant for ISRU applications was an entry in Lunarpedia. In the entry liquid sodium potassium eutectic mixture be used. Due to time constraints robust calculations of this mixture will need to wait until a later date.
After the fact notes.
Realistically most launch solutions for the “near term” of a lunar base are going to focus on water derived fuels or using oxygen to top off the reaction mass of a mission that brings its other chemistry from Earth. Longer term there is a legitimate question on whether the resources needed to refine and build non-HydroLOx engines would be better spent building gas guns, linear accelerators, or spin launchers. That being said it was an interesting learning experience for me to research it and I hope that it might spark something for someone down the line.
As always any questions or feedback is welcome.
-Obie
[1] https://www.researchgate.net/publication/318299632_Heated_Gas_Propulsion_System_Conceptual_Design_for_the_SAMSON_Nano-Satellite_Propulsion
[2] https://arc.aiaa.org/doi/pdf/10.2514/3.8610
[3] Technically small particles of silicon could be used for combustion, but the need to manufacture such fine grains makes this less preferable.
[4] https://ntrs.nasa.gov/api/citations/20090026004/downloads/20090026004.pdf
[5] https://arc.aiaa.org/doi/10.2514/8.7324
[6] https://ntrs.nasa.gov/api/citations/19700026405/downloads/19700026405.pdf page 13 of 45
[7] https://curator.jsc.nasa.gov/lunar/lnews/lnmar97/oxygen.htm Oxygen extraction from lunar samples
[8] https://www.nasa.gov/sites/default/files/atoms/files/2020_aiaa_rampt_multimetallic_compositechambers-final.pdf
[9] https://www.nasa.gov/marshall/news/nasa-3-D-prints-first-full-scale-copper-rocket-engine-part.html
[10] https://ntrs.nasa.gov/api/citations/20150021397/downloads/20150021397.pdf Structural analysis of combustion chambers
[11] 50-150 ppm from solar wind https://ntrs.nasa.gov/api/citations/20190029044/downloads/20190029044.pdf slide 16